Abstract
INTRODUCTION: Recently presented 6-year follow-up data from the CLL14 trial for chronic lymphocytic leukemia (CLL) showed increased overall survival (OS) and progression-free survival (PFS) using venetoclax plus obinutuzumab (VenO) as 1st line treatment when compared to chlorambucil plus obinutuzumab (ClbO) (Al-Sawaf, 2022). However, the higher treatment cost of VenO compared to off-patent chemotherapy requires evaluation of the potential trade-offs of added costs and improved health outcomes to inform decision-making. In Denmark, targeted agents are only reimbursed for treatment-naïve CLL patients with del17p/TP53-mutation. The objective of this study was to investigate the cost-effectiveness of VenO compared to ClbO in treatment-naïve CLL without del17p/TP53-mutation, including the course of disease until death.
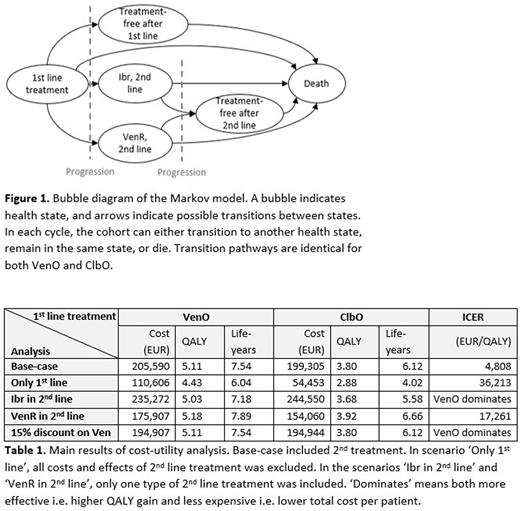
METHODS: A cost-utility analysis was conducted to simulate a hypothetical cohort of CLL patients with characteristics corresponding to the CLL14 cohort without del17p/TP53-mutation receiving either VenO or ClbO as 1st line treatment followed by either venetoclax plus rituximab (VenR) or ibrutinib (Ibr) as 2nd line treatment or no treatment after progression. Second line treatment was assumed to begin one month after progression. Model outcomes were the expected cost, quality-adjusted life-years (QALYs) and life-years (LY) per patient. Outcomes were used to calculate the incremental cost-effectiveness ratio (ICER) i.e. the added cost per extra QALY gained. A Markov model was constructed with a lifetime time horizon (Figure 1) and was set to replicate recently presented data for OS and PFS for 72 months from the CLL14 study. Parametric survival functions were estimated for the remaining life years, assuming a log-normal and exponential distribution for OS and PFS, respectively in the base-case. A Danish health sector perspective was applied with all costs estimated in EUR. Costs and quality of life decrements related to administration of both 1st and 2nd line treatment, as well as adverse event treatment, were included. Best available published data from the MURANO trial (VenR) (Kater et al., 2020) and Danish real-world evidence (Ibr) (Aarup et al., 2020) for 2nd line treatment was utilized to reflect current clinical practice in Denmark. Deterministic and probabilistic sensitivity analyses (PSA) were performed. Due to the absence of an official threshold of marginal cost per QALY in Denmark, results were compared to the British National Institute for Health and Care Excellence (NICE) threshold of GBP 20,000-30,000 (EUR 23,600-35,600).
RESULTS: First-line treatment with VenO provided 5.28 QALY (7.54 LY) for the average patient compared to 3.80 QALY (6.12 LY) in the ClbO group (Table 1). Total costs per patient were EUR 202,821 for VenO and EUR 195,781 for ClbO, resulting in a base-case ICER of EUR 4,808 per QALY. The PSA showed that VenO was recommendable in 97% of the simulations at the lower NICE threshold. Results were robust to the assumptions on choice of 2nd line treatment, while exclusion of all costs and effects of 2nd line treatment showed cost-effectiveness results favoring ClbO. Cost of 2nd line treatment constituted up to 70% of the total patient cost, and especially the use of Ibr in 2nd line was associated with a higher total cost per patient. At a discount of 15% on the list price of venetoclax, 1st line VenO provided savings compared to ClbO.
CONCLUSIONS: VenO was more effective in terms of QALY but at a higher cost than ClbO. VenO was cost-effective with inclusion of costs and effects of 2nd line treatment when using the NICE threshold indicating that VenO may also be cost-effective in Danish, and potentially other European, healthcare settings. While additional sensitivity analyses are awaited, these findings, along with previously published OS and PFS data, suggest that moving VenO into 1st line treatment may be beneficial and cost-effective also for patients without del17p/TP53-mutation.
Disclosures
Slot:AbbVie: Consultancy; Ambu: Current equity holder in private company. Niemann:Janssen: Consultancy; AstraZeneca: Consultancy, Research Funding; Octapharma: Consultancy, Research Funding; CSL Behring: Consultancy; Takeda: Consultancy; Genmab: Consultancy; Beigene: Consultancy; Abbvie: Consultancy, Research Funding. Wulff Risør:AbbVie: Consultancy. Ehlers:AbbVie: Research Funding; Pfizer: Consultancy, Research Funding; Boehringer Ingelheim: Consultancy, Research Funding; Gilead: Consultancy, Research Funding. Rotbain:AbbVie: Consultancy, Other: Travel grants; AstraZeneca: Consultancy, Other: Travel grants.
Author notes
Asterisk with author names denotes non-ASH members.